Spinal cord stimulation (SCS) - Information for patients being considered
Introduction to pain
About pain transmission
Pain impulses travel from the point of origin along pain pathways into the spinal cord and then to the brain.
The brain then recognises these impulses and categorises them as painful and does it’s best to judge their origin. It then forwards impulses to other areas of the brain with the purpose of taking immediate action and making sense of them.
About acute pain
Fast sharp pain is felt during stimulus or injury. Slow dull pain is felt afterwards and serves to promote protective behaviours to enable healing to complete. However, for reasons not yet fully understood, pain can continue beyond the expected healing time, despite efforts to relieve it. This is called chronic pain.
About chronic pain
Chronic pain is unhelpful as it is both unpleasant and exists even when harm or damage is excluded by various tests including medical assessment. When faced with on-going signals in pain pathways, the brain assumes they are a threat and a sign of on-going injury and so a person adopts protective behaviours. Unless rationale and positive thoughts and behaviours (good pain management skills) are adopted, the healed tissue remains sensitised, which fuels this on-going unhelpful pain mechanism. Misdirected problem-solving (poor pain management skills) can develop, leading to further distress and physical disability. Consequent tiredness, boredom, depression and worry make the pain even harder to cope with. The natural analgesic centres in the brain, which thrives on upbeat messages, wane.
About neuropathic pain
Neuropathic pain is a type of unhelpful chronic pain originating in the nervous system and often resistant to conventional treatment (including medications and good pain management skills) but can be eased by spinal cord stimulation (SCS).
Introduction to spinal cord stimulation (SCS)
About SCS
Naturally occurring electricity has been used to treat pain for thousands of years. In 1971 the first SCS was implanted delivering low-voltage electrical currents to the back of the spinal cord where the ‘spinal pain gates’ lie, providing pain relief. We believe SCS recruits natural pain relieving mechanisms at a spinal cord level and via longer pathways which stimulate pain relieving centres in the brain. It offers no cure but can offer people with neuropathic pain a degree of relief that enables them to become more active, enjoy better sleep and possibly reduce their pain medications. The Walton Centre has implanted stimulators for over 35 years.
About the multidisciplinary assessment prior to trial
You will have been reviewed by one of the pain doctors or neurosurgeons for initial assessment regarding suitability of SCS and then referred for this detailed assessment. A specialist team experienced in all manner of pain management carries out a comprehensive assessment of your pain and its effect on your day-to-day life. They will be able to answer your questions or reassure you about any concerns you may have and will provide further education and information around SCS. The assessment team may recommend additional treatments to your doctor so as to optimise your chances of success from SCS or minimise any risks from this treatment or suggest alternatives. Your case may be referred for further discussion among the wider multidisciplinary team so your doctor can hear other’s views regarding the suitability of this treatment or any other management options for you.
The reason for a SCS trial
A low risk trial of SCS is usually carried out before considering a permanent implant (the latter poses rare but significant risks) because despite careful assessment and selection, not every person feels the same degree of pain relief, initially or in the longer term. At your consultant’s discretion, they may offer to internally secure a percutaneous wire/lead or surgically place a flat paddle lead to be connected to an external trial unit for a period. This same lead can then be attached to an implanted IPG minimising discrepancy between the trial and permanent SCS sensation. A genuinely successful trial predicts similar relief from a subsequent permanent device in the short-term but does not guarantee it in the long-term. Of course the placebo effect (trial being positive for poorly understood reasons) cannot be excluded.
The SCS trial
The day of your arrival to hospital
You will not need pyjamas / nightdress, but it may be useful to bring a dressing gown and slippers. Your consultant conducting the trial will give you instructions regarding fasting and fluid intake. You will be required to return to the ward (usually Jefferson or Chavasse) for a number of days during your week-long trial. Hotel accommodation can be provided for patients who do not live locally, or in some cases you will require an inpatient stay.
In theatre
The procedure is performed in theatre to minimise the risk of infection. You will have a cannula in your vein to administer antibiotic and any other medication as may be needed. Routine checks will be carried out to ensure that you are having the correct procedure. You will then be assisted to lie face-down on the theatre table with a cushion under your stomach. Antiseptic will be applied to the area where the SCS lead will be inserted to minimise risk of infection. An X-ray machine will be positioned over you so the doctor can accurately visualise the space between the bones in your spine. Local anaesthetic will be injected to numb the skin. A needle will be inserted under X-ray guidance until it reaches the (epidural) space around the spinal cord. The SCS lead is then introduced under X-ray guidance into the epidural space. It will be positioned carefully and activated several times until you tell the stimulator operator that the tingling sensation is pleasant and overlapping most or all of your pain area. The stimulation is stopped and the hollow needle is then removed carefully to leave the lead in place. The process, including securing the lead with a dressing and sometimes a stitch, usually takes 30-90 minutes. Rarely, the procedure is abandoned if unsuccessful, or if the patient cannot tolerate it because of painful sensitivity of the spine.
On return to the ward
As part of their daily rounds, a member of the pain team will visit you to reconnect the trial stimulator device and extension, fine tune it and provide you with instructions on its use. We will ask you to completed pain and activity diaries and exercise good wound care. (You must keep the dressing dry by not showering or immersing that area of your body for the duration of the trial and for a couple of days beyond). We ask you to keep taking your pain medication and use simple painkillers such as Paracetamol for the needle site pain and take reasonable precautions to reduce the risk of a pain flare-up as well as accidental removal of the SCS lead. If the dressing becomes loose, call us.

The remaining days of your trial
You will be reviewed at intervals by the Nurse, Physiotherapist, Pain Consultant, Registrar or Neurosurgeon to evaluate improvements and check your wound and dressing. An X-ray of the lead’s position will be taken to provide the implanters with a target for the permanent lead. In case you are added to the waiting list for the permanent implant, a physiotherapist will measure your physical capabilities e.g. walking, stair climbs with your stimulator off, against which your future physical improvement can be compared.
Possible complications of the trial
In some, the location and strength of stimulation can be extremely changeable, even between minor adjustments of posture, making stimulation difficult to tolerate and evaluation of effect difficult. Often, those who experience this positional change during the trial, experience it from the permanent system as well. Despite taking precautions, the lead can drift and unless corrective programming is successful, stimulation can be lost. It is not uncommon to feel back or neck pain from the insertion and repositioning of the needle, especially if it was a difficult placement, but it usually settles in a day or two and eases with simple pain killers. Rarely a small leakage of spinal fluid from around your spinal cord can occur during the procedure in theatre resulting in a headache and abandoning of the procedure – the trial will be rescheduled. In rare cases this headache can persist but this is treatable with another injection.
The final day of your trial (removable trial lead)
The pain team, usually including the neurosurgeon that implants, will draw upon their experience and if your response to the trial forecasts a good effect with a permanent device, they will meet with you at a mutually convenient time and explain the operation including the risks and benefits, answer any questions and add you to their waiting list. If you wish, we can show you the demonstration models of the implant again. Before you leave hospital, one of the team will remove the lead on the ward, check the insertion wound and apply a small dressing. You will be asked to check that your dressing and wound is dry thereon and contact us immediately if any signs of infection develop (redness, swelling, pus discharge). You can resume normal activities like showering and swimming when the wound is healed over (normally 48 hours after lead removal).
Various manufacturer's trial kit
The permanent implant
Pre-operative appointment
This appointment is sent out one to two weeks to your prior admission date and lasts for 45 minutes but please be prepared to stay longer. You will be seen by a Neuromodulation nurse specialist who will ask you a series of health related questions to ascertain that you are fit and well enough for the general anaesthetic. You will also have a series of blood tests, blood pressure, height and weight measurements and urine tests and possibly an ECG (heart trace test). Please bring any relevant history and current medication. This will also be a question and answer opportunity.
Hospital stay
The stay for this procedure is usually three days, starting the day before surgery and normally takes about two hours in theatre.
The operation
- CLOSED: Under local anaesthetic, your pain consultant will insert the percutaneous lead through a needle under XR control and then anchor it. At this point or after a trial period they will then anaesthetise the skin of the upper buttock and cut a pocket for the IPG and then thread the connecting extension cable through fatty tissue under the skin.
- OPEN: Under general anaesthetic your neurosurgeon will make a midline spinal incision below the intended location of the electrode then remove enough bone and ligament tissue to place the permanent electrode flat on the surface of your cord. The extension cable is then passed under the skin and connected to the IPG which is again implanted under the skin usually in your upper buttock. Sometimes an extra extension lead is used, requiring an additional small incision under your shoulder blade. It is not uncommon to be able to feel and sometimes see the outline of the IPG and cables under the bare skin which is of no consequence.
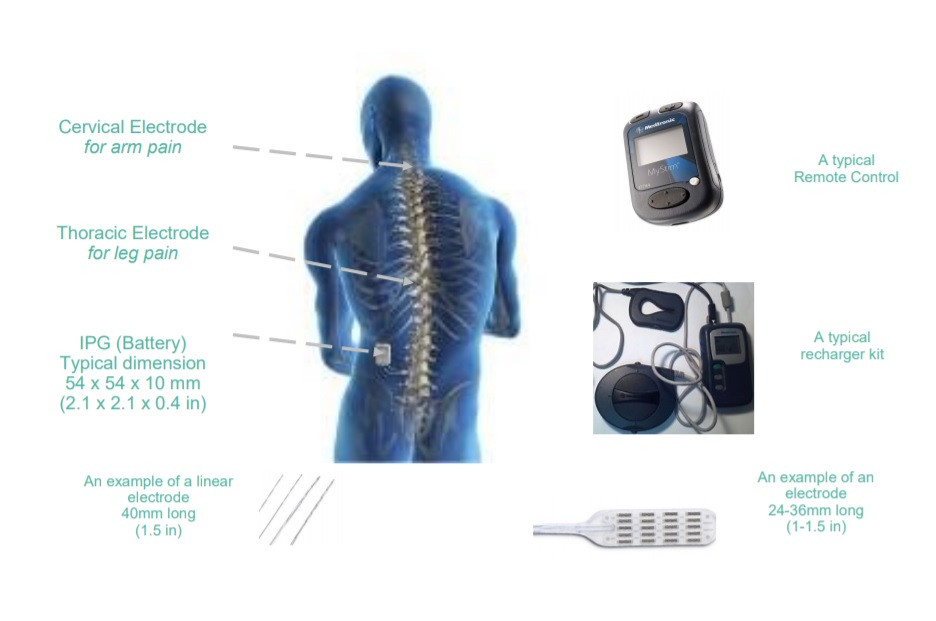
After the operation and discharge
Hardware
The nurse or physiotherapist will switch your device on as soon as is appropriate. Optimal programming is rarely achieved immediately post-op (optimal tuning is usually reached after a six months settling period). You will also be shown how to use your remote control (and re-charger if your surgeon has chosen to implant you with a rechargeable version).
Post-operative pain
Please note a minority of people will experience IPG site pain or spinal wound pain in the absence of anything medically wrong. This can sometimes be severe. If it is severe then local anaesthetic patches may be prescribed initially however sometimes this pain can not be relieved by this means. We usually give the area six months to settle before considering any surgical intervention (e.g. repositioning or removal).
Discharge procedure
You will be ready to go home when you are: a) medically fit; b) confident in using your system; c) feeling acceptable coverage of stimulation; d) fit to cope at home. After your check X-ray, the ward will arrange for your stitches or staples to be removed 7-10 days after your surgery, by your Practice Nurse or District Nurse or you can attend a Walk-in Centre 10 days post-op for this. It is common for the dressing placed in theatre to remain on for the post-operative period.
After you leave hospital
It is important to keep your wounds dry and clean, until they have healed and the stitches or staples have been removed. If you spot any signs of infection such as redness, swelling or leakage from your wound sites, contact us immediately (rather than your GP) using the telephone numbers provided. You will be given a post-op follow-up clinic appointment 3-6 weeks after implant to i) check that your wounds have healed well, and ii) assess whether you also need a separate session to tune your stimulator in our programming clinic. Do contact us if you haven’t received a post-operative follow-up appointment in the post. Your next scheduled appointment will be six months after implant to both adapt your medications as appropriate and evaluate your progress. If you require attention to your device e.g. because you have lost stimulation, please contact us prior to attending these follow-up clinic to book you into our programming clinic. You will then be reviewed at intervals depending on the need for reprogramming, monitoring IPG life, medication reviews and physical progress re-assessment. However, please contact the Neuromodulation Team if you have any queries or concerns. Some appointments may be exclusively for progress review including physical capacity measures and questionnaires required by commissioners of our services; these will only be done with your consent.
Following discharge from hospital with an SCS we advise that you have a period of rest, with gentle mobilisation and regular movements for best healing. Heavy lifting, twisting and bending should be avoided in the early weeks. Normal activities of daily living, i.e. washing, dressing, etc. can be resumed immediately post-op. However, we recommend no showers or baths until after your sutures/clips have been removed and the wound areas are healed.
We expect that the procedure will eventually enable you to perform normal daily activities easier, however please be sensible and avoid activities that could damage and/or displace the system (sudden, excessive or repetitive bending, stretching, twisting, bouncing or impact).
Wound management
If at any time you have any concerns regarding your wounds, please contact the Neuromodulation Team in the first instance. Due to having an implanted IPG and electrode, it is important to contact us as early as possible with any signs of infection. If infection does develop, it can occasionally lead to the system having to be removed whilst the infection is treated and a new system implanted at a later date.
Signs of infection include:
- Redness and/or swelling around the wound areas
- Leaking from wounds
- Pain around wound areas
- Feeling feverish or unwell (flu-like symptoms)
Work
When, and in what capacity, you return to work will largely depend on what your job involves. It is advisable to discuss this with us, if it’s an issue.
Driving
You should not drive with your SCS switched on. These devices can occasionally give a small jolt (you may find this happens with positional changes) and these could be distracting whilst driving.
Which medical treatments or investigations are hazardous to me to have with the stimulator and require your prior permission?
- MRI scans (used in X-ray departments). There are rare exceptions so please contact us or ask your medic to do so.
- Ultrasound (used in X-ray and physiotherapy departments)
- Short wave diathermy (used in physiotherapy departments)
- Electrocautery (used during surgery)
- Radiotherapy (used in X-ray departments for cancer treatment)
- Lithotripsy (used to break up bladder, kidney and gallstones)
- Bone growth stimulators (internal or external)
Possible complications
- Failure or breakage of part of the system (~10%) and/or significant drift of the electrode (~5%) will require corrective surgery. Wound infection (~3%), reaction to metal in the implant (~0.1%), damage to the spinal cord (~0.16%), meningitis (~0.16%), bleeding (~0.5%) or infection around the spinal cord are rare complications but could be life threatening.
New types of chronic pain
- i) spinal pain close to the surgical site
- ii) IPG pocket-site pain. Rarely these pains can be as severe or more severe than the original pain for which the SCS is implanted, and then have a significant impact on quality of life. Headache due to a small leakage of fluid from around your spinal cord can occur and last a few days. In rare cases (~0.16%), this may become persistent.
Device identification
If not already, you will receive an ID card soon from the manufacturer of your implanted device. This should be carried with you at all times. It carries your details and will be required when going through security checks at airports as you must avoid walking through the full body scanners. Hand-held scanning devices may be used on you.
All risk figures correct at the time of printing.
Signs of infection include:
- Redness and/or swelling around the wound areas
- Leaking from wounds
- Pain around wound areas
- Feeling feverish or unwell (flu-like symptoms)
Equipment
It is expected that you will look after the kit provided as they are robust but not indestructible. In the event of loss or damage to equipment or if you are experiencing a technical problem with the programmer or recharging device, please contact the Neuromodulation team (not the manufacturer) without delay.
Frequently asked questions
Is SCS safe?
There have been no reports of adverse effects of SCS electrical stimulation on animal or human tissue. Patients with a confirmed allergy to metal (e.g. nickel), are unsuitable for SCS treatment. If you think you might have a metal allergy we can arrange allergy testing for you, however this will not predict your response to long-term exposure.
What about the evidence for long term efficacy?
A study by North in 2008 demonstrated significant pain relief after two years of SCS for persistent leg pain after back surgery. Kumar in 2005 demonstrated better than 50% relief after an average of eight years implanted, in 75% of a group with a mix of pain conditions In the event of loss of effect from a perfectly functioning SCS, your consultant(s) will explore treatment options that are available. Under these circumstances any part of the system can be removed but only if is it proving to be a nuisance or preventing investigations.
How long will it take me to get back to normal activities?
- These timescales are only a guide, so if concerned please consult your clinician
- Occasional light lifting……3 weeks after surgery
- Work and driving………… 4 - 6 weeks after surgery
- Heavy lifting…………….. 12 weeks after surgery
How long does the power last in the IPG?
The IPG is a self-contained unit comprising of the power unit and the electronics, so is replaced as one (usually under a local anaesthetic). Their lifespan is dependant on many factors but the average is four years (ranging between three months and 10 years depending on individual demands and biological factors). Standard and rechargeable IPG’s and the various makes have their pros and cons so their selection is made on a case-by-case basis at the discretion of the neurosurgeon.
Can magnetic fields and electrical equipment affect the stimulator?
Most household electrical equipment in good working order and properly earthed will not interfere with your stimulator. Some stimulators can be switched off in an emergency with a magnet, so it is best to keep the implanted IPG at least 10 inches away from i.e. magnetic strips in freezers and fridges. Some household devices such as computer disk drives, radios and any type of phone are best kept six inches (15cm) from the implanted IPG. Household power tools are best kept at arms length. Keep at least 25 ft. (8m) from electric substations. Large industrial equipment may reset stimulators to factory settings which can be resolved by your clinician. Be warned arc-welders and security devices may cause a shock or a jolt, even when the stimulator is off. Please consult your user manual for more information.
Which medical treatments or investigations interfere with the stimulator?
It is important to let your health care professional know that you have a stimulator before you attend for any treatment or investigations. Cardioversion / defibrillation / pacemakers (to treat irregular heart rhythms) can interfere with the normal functioning of your stimulator. If these cardiac devices are required after implant of your SCS then this will require specialist staff to manage that pre-operatively.
Which medical treatments or investigations are safe to have?
- Standard X-rays are safe
- TENS machines are safe; the pads should be at least 4 inches (10cm) away from any part of the implanted system.
- Mammograms are safe; however it is best to turn your stimulator off.
- Laser treatment is safe as long as is not applied directly over any part of the implanted system.
- Dental drills, mixers and ultrasonic de-scalers are safe as long as they are in good working order and regularly serviced and kept at least 6 inches (15cm) away from any part of the implanted system, however it is best to turn your stimulator off.
Will security devices interfere with the stimulator?
Transmission from some security devices in shops and airports may cause random stimulation or surges, so approach these devices slowly and if you sense your stimulator is being affected, switch it off whilst you pass through. The implanted devices can trigger security alarms so have your ID card at hand to show staff.
Can I go on board a plane?
Commercial flights will not affect your stimulator; however you will need to prove to security staff that you have an implanted device by showing your ID card and remote - they will take you through the alternative security screening.
When can I return to sport?
You can return to activities like swimming when your wounds have healed. You should gradually build back up to non-contact sports over the six to eight weeks postoperative period. Contact sports should be avoided as they could lead to electrode damage and IPG site disruption.
This leaflet is only a guide. For clarification and current information, please consult your user manual issued to you with your implant.
Any questions?
In this leaflet we hope you find all the information you need following discharge from The Walton Centre. However, if you have any further questions please do not hesitate to contact us. Our contact numbers are included in this leaflet .If they are of an urgent nature, or you can discuss them at your post-operative follow-up appointment.
Routine contact numbers
- Monday to Friday 0800 – 1700 hrs
- Appointments: 0151-529-5322
- Clinicians: 0151-529-5644
- Emergency out of hours contact numbers: Evenings, weekends, bank holidays: 0151-525-3611. Ask for the Senior Nurse bleep holder explaining that you have a spinal cord stimulator implanted and what your concerns are.
- Last Updated:01 April 2024
- Review Date:01 April 2028
- Author:Mark Draper
- Summary:
Information for patients being considered for spinal cord stimulation (SCS)